Understanding the Periodic Table Basics: Zn Periodic Table Drawing Easy
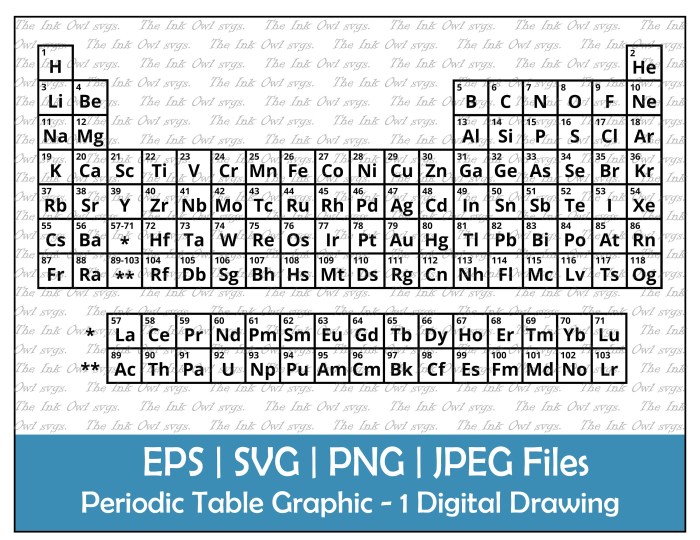
Zn periodic table drawing easy – The periodic table is a powerful tool that organizes all known chemical elements. Understanding its structure is key to understanding chemistry. It arranges elements based on their atomic structure and recurring chemical properties, revealing patterns and relationships between them.
Mastering a simple Zn periodic table drawing is a great starting point for visualising chemical elements. Understanding the structure of atoms helps when considering the microscopic world, such as when drawing the shapes of microorganisms; for example, you might find a helpful guide on bacteria cold drawing easy illustrations. Returning to the macroscopic, accurate representation in your Zn periodic table drawing hinges on understanding atomic structure and periodic trends.
The periodic table arranges elements in rows (periods) and columns (groups). Elements in the same group share similar chemical properties because they have the same number of valence electrons – the electrons in their outermost shell. Elements in the same period have the same number of electron shells.
Atomic Number and Atomic Mass
The atomic number of an element represents the number of protons in the nucleus of an atom of that element. It uniquely identifies each element; no two elements have the same atomic number. For example, the atomic number of hydrogen is 1, meaning each hydrogen atom has one proton. The atomic mass, on the other hand, is the average mass of all the isotopes of an element.
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. Atomic mass is usually a decimal number because it accounts for the relative abundance of different isotopes.
Electron Shells and Valence Electrons
Electrons orbit the nucleus in energy levels called electron shells. The first shell can hold up to two electrons, the second shell up to eight, and so on. Valence electrons are the electrons in the outermost shell. These electrons are involved in chemical bonding, determining an element’s reactivity and the types of compounds it can form. Elements in the same group have the same number of valence electrons, leading to similar chemical behaviors.
For example, elements in Group 1 (alkali metals) all have one valence electron, making them highly reactive.
Simplified Visual Representation of an Element’s Structure
Let’s consider a simplified model of a Lithium atom (Li), which has an atomic number of 3. Imagine a central nucleus containing 3 protons (positive charge) and 4 neutrons (neutral charge). Surrounding the nucleus are three electrons (negative charge) orbiting in shells. Two electrons occupy the first shell closest to the nucleus, while the remaining single electron occupies the second shell.
This single electron in the outer shell is the valence electron, responsible for Lithium’s reactivity.
A simplified visual representation: Imagine a small, dense circle (the nucleus) containing three “+” symbols (protons) and four neutral symbols (neutrons). Around this nucleus, draw two concentric circles. The inner circle contains two “-” symbols (electrons), and the outer circle contains one “-” symbol (valence electron).
Easy Drawing Techniques for the Periodic Table
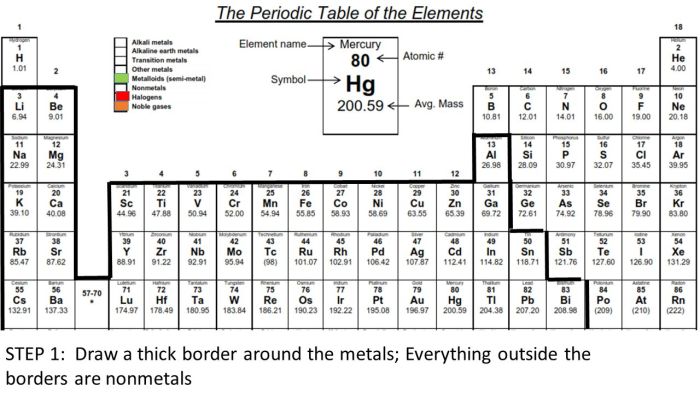
Drawing a periodic table doesn’t require artistic mastery; a few simple techniques can produce a clear and informative representation. This section will explore different methods for creating your own periodic table, focusing on ease and clarity. We’ll also discuss the advantages of using different tools and color-coding strategies.
Methods for Drawing a Basic Periodic Table Layout, Zn periodic table drawing easy
Three common methods simplify creating a periodic table: using grid paper for precise alignment, employing a freehand sketch for a more organic feel, and leveraging digital tools for flexibility and ease of correction. Each method offers unique advantages depending on your skill level and desired outcome.
- Grid Paper Method: This method provides excellent accuracy and alignment. Using grid paper ensures that the elements are neatly arranged and the table maintains its characteristic rectangular shape with consistent cell sizes. This is particularly useful for beginners who want to ensure perfect proportions and spacing.
- Freehand Drawing Method: This approach allows for more creative freedom. While requiring more skill and potentially leading to inconsistencies in cell sizes and alignment, it offers a more personal touch. It’s suitable for those comfortable with sketching and prioritizing a less rigid design.
- Digital Drawing Method: Software like Microsoft Word, Google Docs, or dedicated drawing programs provide a highly flexible option. Errors can be easily corrected, and the final product can be easily shared or printed. This method offers the best combination of precision and ease of modification.
Grid Paper versus Freehand Drawing
Grid paper offers superior precision and consistency, especially for beginners. The structured grid helps maintain accurate proportions and spacing between elements. Freehand drawing, while allowing for more artistic expression, may result in a less uniform and potentially less accurate representation, particularly if the drawer lacks experience. The choice depends on the desired level of accuracy and artistic freedom.
Color-Coding for Element Groups
Color-coding is crucial for enhancing the visual appeal and understanding of the periodic table. Different colors represent various element groups (alkali metals, alkaline earth metals, halogens, noble gases, etc.), making it easier to identify patterns and relationships. For example, alkali metals could be consistently represented in a light purple, while halogens might be a vibrant green. This visual distinction aids in quickly identifying elements with similar chemical properties.
Step-by-Step Guide: First 20 Elements
This simplified periodic table will focus on the first 20 elements, providing a manageable introduction to the process.
- Preparation: Gather grid paper (recommended for beginners), a pencil, eraser, and colored pencils or markers.
- Layout: Draw a rectangle on your grid paper, dividing it into approximately 2 rows and 10 columns. Adjust the number of columns as needed to accommodate the elements.
- Element Placement: Write the element symbols (H, He, Li, Be, B, C, N, O, F, Ne, Na, Mg, Al, Si, P, S, Cl, Ar, K, Ca) into the appropriate cells, following the order of the periodic table. Hydrogen (H) and Helium (He) are often placed separately at the top.
- Color-Coding: Assign colors to the different groups. For example, use a light blue for alkali metals (Li, Na, K), a light green for alkaline earth metals (Be, Mg, Ca), and so on.
- Refinement: Once all elements are in place and colored, carefully erase any pencil marks and review the layout for any necessary adjustments.
Q&A
Can I use this method to draw the entire periodic table?
While this guide focuses on a simplified approach, the techniques can be adapted to draw the complete periodic table. It will require more time and space, but the principles remain the same.
What kind of materials do I need?
You’ll primarily need paper (grid paper is recommended), pencils, colored pencils or markers, and a ruler. Optional materials include a compass for creating circles representing atoms.
Are there online resources to help with element information?
Yes, many websites and online databases provide detailed information on elements, including their properties, symbols, and atomic weights. Check reputable sources like the Royal Society of Chemistry or the National Institute of Standards and Technology.
